DAIMON—An Original Data Mining System Pharmaceutical Production
The pharmaceutical industry handles a vast amount of data, not only in research and development but also in production. Producing Active Pharmaceutical Ingredients (APIs) involves chemical reaction with structural change and purification. Furthermore, the manufacturing process of products using APIs includes lots of processes such as uniformly mixing APIs, tableting, all of the processes are strictly implemented and controlled based on data.
These highly rigorous production processes are necessary for Astellas to deliver on our commitment to provide a stable supply of high-quality pharmaceuticals to patients. And as we endeavor to maintain and improve those processes, Astellas independently developed DAIMON, a state-of-the-art data mining system.
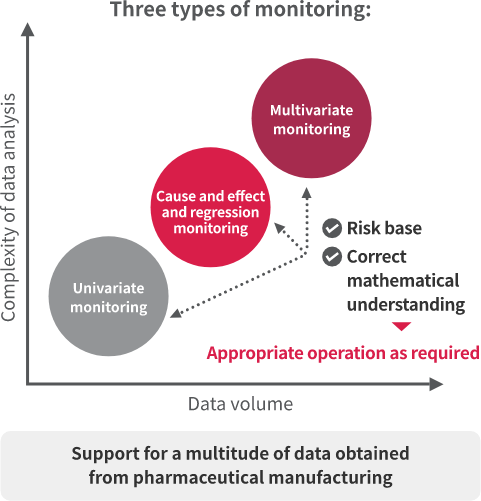
The DAIMON data mining system for manufacturing incorporates (1) univariate monitoring, (2) cause and effect and regression monitoring, and (3) multivariate monitoring. Significant time reductions in data analysis can be achieved through the appropriate operation of these three monitoring types, according to the volume of data and the complexity of the analysis.
DAIMON's strength lies in a continuous cycle of knowledge acquisition. Starting with monitoring trends, the cycle then detects fluctuations/predicts risks, investigates causes/prevents problems and improves understanding of products and processes. Through this cycle, it is possible to promptly react to quality or production trouble and prevent such troubles in advance, thereby realizing a high manufacturing level and a stable supply of pharmaceutical products.
Development of Formulation Design AI
When developing tablets for small molecular compounds, the composition of excipients (formulation) added to the drug substance is important in order to achieve the quality target product profiles (QTPP: ease of dissolution, hardness, etc.). A lot of experiments are generally required to find the optimal formulation. If we can streamline these experiments and develop high-quality tablets in a short period, we can deliver medicine to patients faster. Since 2018, Astellas has been collaborating with Hitachi, Ltd. and developing Formulation Design AI using data accumulated internally to date.
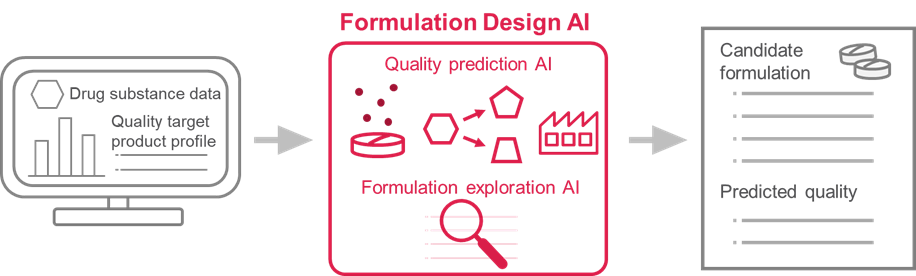
Formulation Design AI consists of AI for predicting tablet quality and AI for exploring optimal formulation. When drug substance data and QTPP are input into Formulation Design AI, it comes up with potential candidate formulations that could meet the target and their predicted tablet quality.
The QTPP to be input includes dissolution (the rate at which the drug substance dissolves from the tablet), stability (the extent to which the drug substance in the tablet changes during storage due to decomposition, etc.) and manufacturability (whether the tablets can be molded to the desired hardness without any manufacturing difficulties). For tablet development, Formulation Design AI is expected to enable simultaneous optimization of multiple quality items, which will help us to deliver medicines to patients faster.
The development of an application with a user-friendly interface has been completed, and we have started applying it to the formulation design of actual developmental compounds. Since data volume greatly contributes to the accuracy of AI, we are building a system that can continuously collect and organize internal data, and we will continue to improve the accuracy of Formulation Design AI.
Related links