Astellas is committed to creating innovative drugs with cutting-edge science that can address areas of high unmet medical needs. We aim to help patients who have no treatment options, or do not respond adequately to existing treatments.
We strive to improve and enhance quality throughout the product lifecycle, not only in drug discovery, development, production, and commercial but also in post-marketing surveillance, safety information collection, and our collaborative work with medical professionals. Astellas conducts business in more than 70 countries and regions around the world, and in addition to complying with each country and region’s laws, regulations, and guidelines, we have established a system where all employees engage in business activities with high ethical standards and a broad perspective.
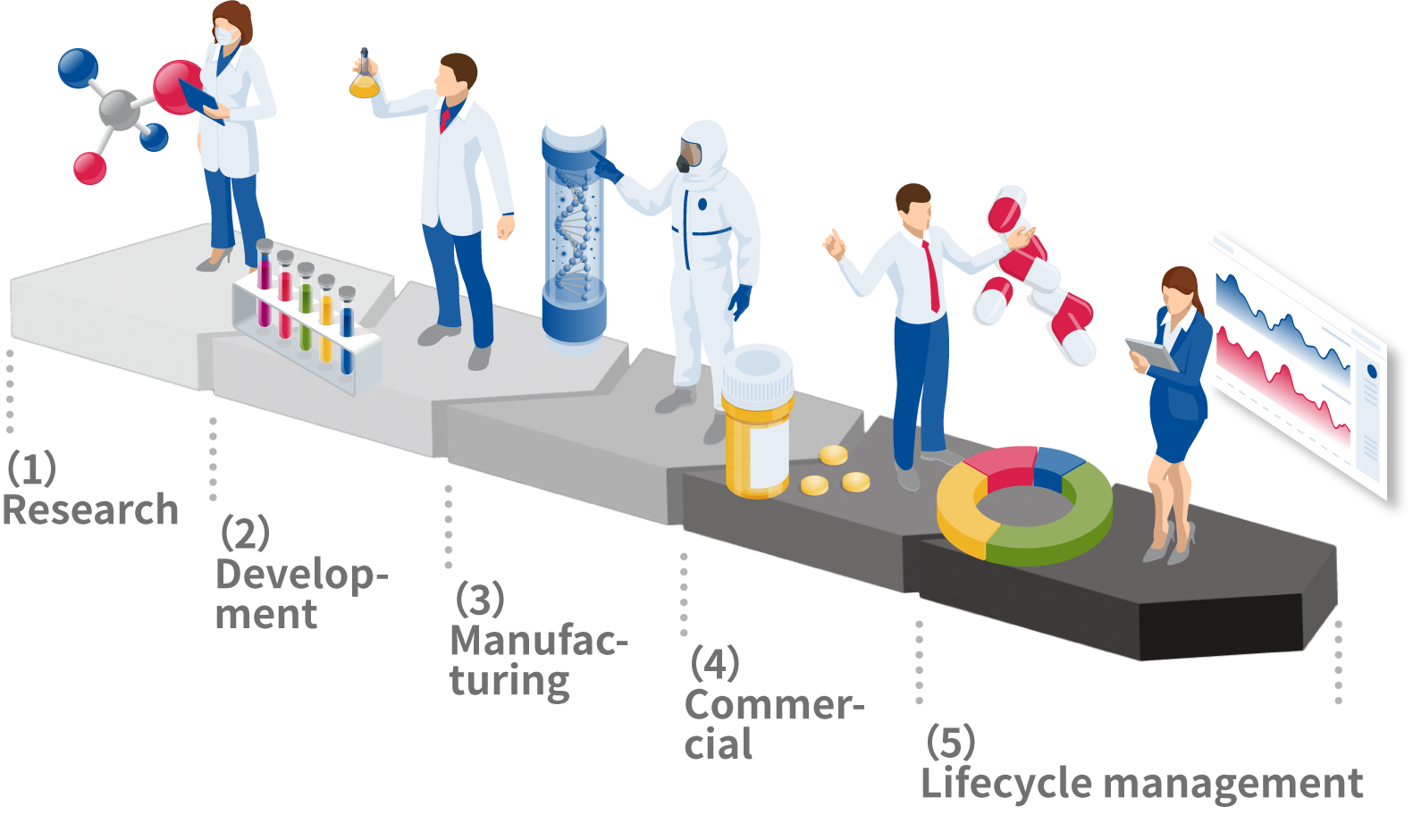
(1) Research
Our comprehensive drug discovery spans from basic scientific to pre-clinical research. Alongside the work of our in-house research team, we also collaborate with various external partners to explore a range of drug discovery opportunities and create clinical development candidates.
(2) Development
We formulate clinical development plans to evaluate the efficacy and safety of clinical development candidates created through drug discovery research. During the clinical trial stage, we investigate and study administration methods and safety, compile data, and file an application for manufacturing and marketing approval. Only after regulatory review and approval is a drug ready for prescribing to patients.
(3) Manufacturing
We design production methods and formulations and produce pharmaceutical products based on established manufacturing processes. We ensure a stable supply of high-quality pharmaceuticals by procuring the raw materials from which pharmaceuticals are made, understanding the manufacturing process, and thoroughly controlling facilities and quality.
(4) Commercial
We provide information to physicians, pharmacists, and other medical professionals to ensure the correct use of our pharmaceuticals. We also collate all information reported after a drug is prescribed to patients, including details on efficacy, quality, side effects, and any other safety-related issues.
(5) Lifecycle Management
We utilize the information we procure from medical professionals, external researchers, and medical experts to explore new drug discovery opportunities, improve products, and further promote appropriate product use.
Many people worldwide have difficulty accessing healthcare due to a lack of available treatments, poverty, healthcare system challenges, and insufficient healthcare information. Astellas regards "Access to Health" as a serious challenge and is leveraging its capabilities and technology to provide various initiatives to deliver necessary medicines and healthcare solutions to those who need them.
Developing new healthcare solutions beyond medicine
At Astellas, we recognize that we can take different approaches from treatment with prescription drugs (Rx) to deliver VALUE to patients across the whole patient journey from diagnosis and prevention to treatment and prognosis. We call this initiative our Rx+® business. By leveraging Astellas' expertise and knowledge cultivated through our Rx business, we integrate innovative medical technology with cutting-edge technology in different fields to create new services and solutions.
Accelerating our business through DX strategy
Astellas is driving digital transformation (DX) throughout our business activities. Here, we introduce some of our key DX initiatives.