Translational research acts as the bridge between non-clinical studies and clinical outcomes. Multiple efforts are advancing to accurately predict clinical outcomes in patients based on non-clinical data, and to incorporate clinical information into non-clinical research. Leveraging insights from patients in early-stage research will help accelerate the drug discovery process and increase the probability of success.
One of Astellas’ distinguishing features is to perform pharmacokinetic (PK) and pharmacodynamic (PD) analyses with high reproducibility across different modalities and to predict drug candidate behavior in the human body. We are also pioneering the use of microphysiological systems and human clinical samples to validate concepts for drug candidates. Additionally, we integrate information into quantitative models, simulate complex biological systems and build development strategies tailored to drug candidate characteristics.
Astellas fosters a collaborative environment for both internal and external researchers, combining multiple technologies to advance translational research initiatives.
PK/PD Analysis
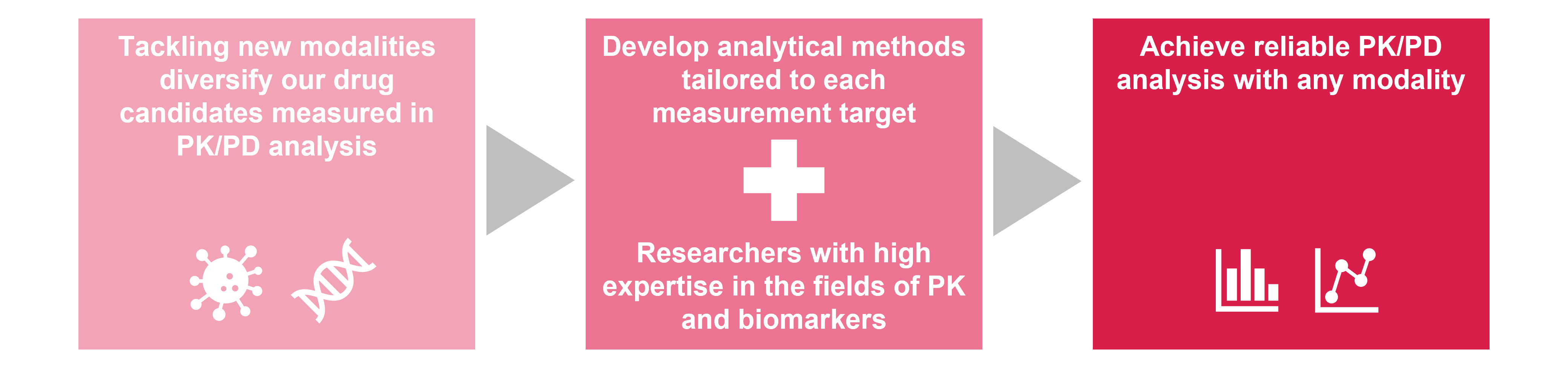
PK/PD analysis estimates drug candidate behaviors in the human body and the efficacy brought by these behaviors. Our efforts in tackling new modalities, in addition to small molecules and antibodies, diversify our drug candidates to be measured in PK/PD analysis and biomarkers to evaluate their mechanism. It is necessary to develop analytical methods that provide highly reproducible results, by considering methods and protocols tailored to each measurement target.
Astellas researchers have high expertise in the fields of PK and biomarkers. By leveraging our diverse knowledge and skills, we analyze PK/PD to produce reliable results with new modalities.
This advanced PK/PD analysis enables us to understand complex biological systems. We leverage these insights to develop therapeutic strategies including optimal dosage and combination methods, and to plan clinical trials.
Pioneering Microphysiological System
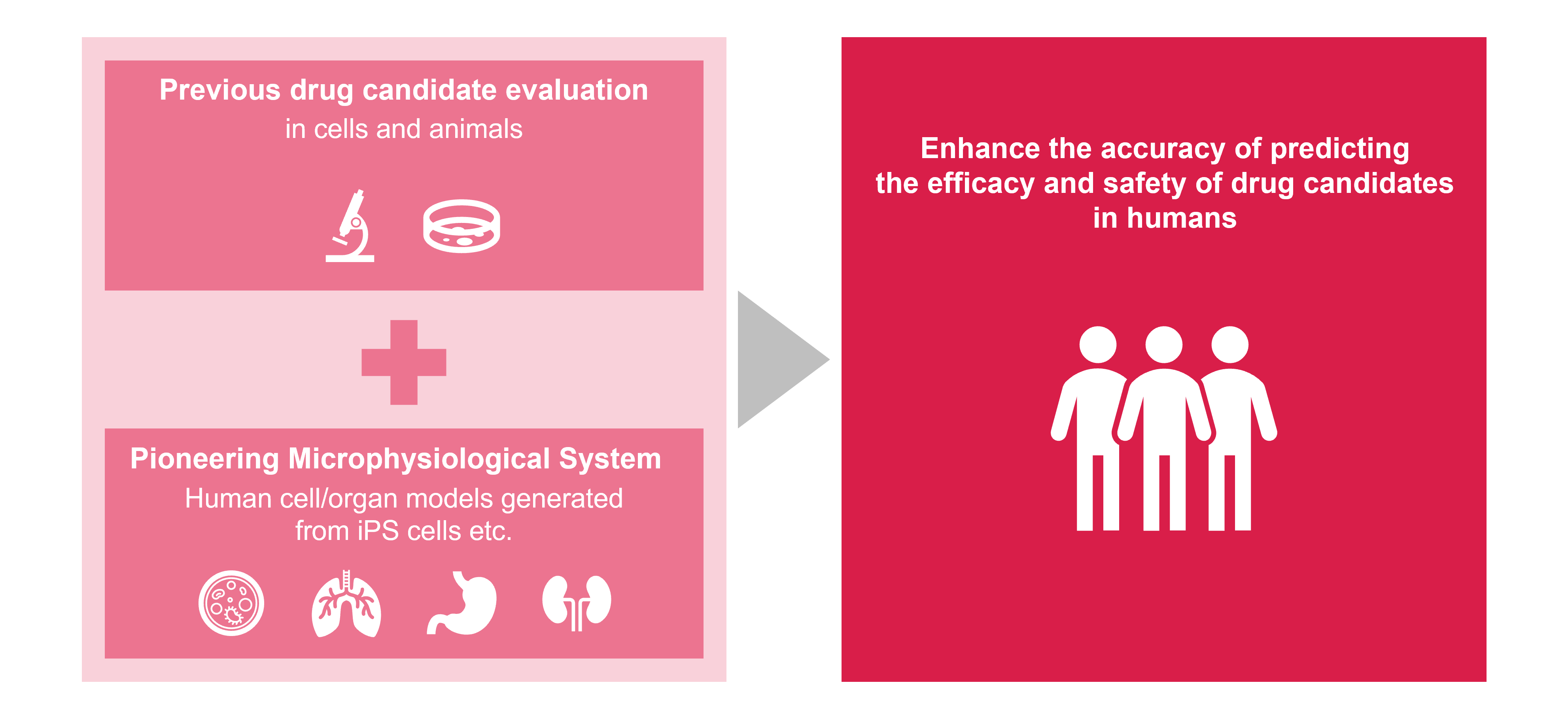
There has been growing interest in the microphysiological system that recapitulates human tissue structure and function in vitro, while non-clinical studies have traditionally relied on animal models. We anticipate this approach will enhance the accuracy of predicting the efficacy and safety of drug candidates in humans, as well as giving mechanistic insights to our drug candidates, highlighting their value to patients. The Food and Drug Administration (FDA) Modernization Act 2.0 in the U.S. has paved the way for regulatory approval of drugs evaluated with alternative models to animal testing, implying the potential for greater adaptation of these models in the future.
Astellas is actively advancing the development and use of the microphysiological system in collaboration with academia and biotechnology companies. In addition to generating highly differentiated cells from induced pluripotent stem (iPS) cells, we are also working to create models that represent complex biological systems, including cell-to-cell interactions, by integrating human cells and unique culture platforms. As part of this effort, we have successfully developed a model* that partially mimics the functionality of renal proximal tubules for kidney injury research. Additionally, we have also established our own protocols to evaluate hepatotoxic potential of our drug candidate in humans, where traditional animal models often fail to detect side effects. As these complex models require significant work even for highly skilled researchers, recent investments in robotics and lab-automation greatly contribute to development of high-quality and robust models and running assays.
* Marianne K Vormann et al. Modelling and Prevention of Acute Kidney Injury through Ischemia and Reperfusion in a Combined Human Renal Proximal Tubule/Blood Vessel-on-a-Chip, Kidney360 2021 Nov 4;3(2):217-231
Mathematical Model Analysis to Understand and Predict Complex Biological Systems
During drug discovery and its research stages, it is critical to understand the biological system associated with the disease, the behavior of drug candidates in the human body and the mechanism of action, in order to estimate the efficacy of the drug candidate in humans. However, biological systems are complex and prediction is challenging, especially for new modalities.
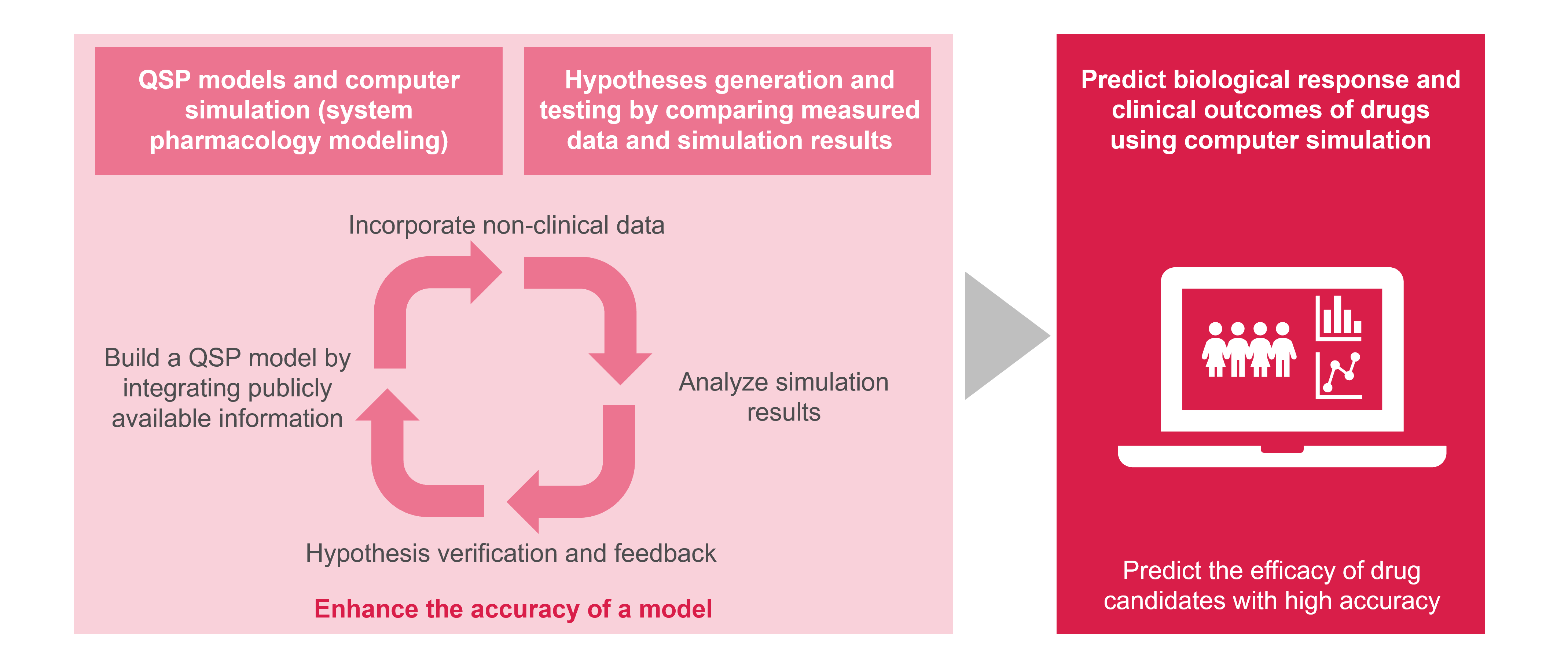
For these complex predictions, we use Quantitative Systems Pharmacology (QSP) to mathematically model the interaction between the biological system of interest and the drug candidates. The QSP model builds on integration of non-clinical data and publicly available information for computer simulation.
At Astellas, we continuously integrate data from early drug discovery into our QSP models. Running a cycle of hypothesis generation and testing from an early stage enhances the accuracy of the forecasting system we create. Our strength lies in timely incorporation of information obtained through cutting-edge biomarker measurement technologies and microphysiological systems into our models, enabling us to predict drug efficacy and providing us with exciting opportunities for growth and innovation.