Allogeneic Cell Therapy
We are working on integrating cell science and genetic science to develop the cell therapies of the future. We work to apply the latest technologies to the development of allogeneic cell therapies which allows us to develop “off-the-shelf” cell therapies that can be used in any patient.
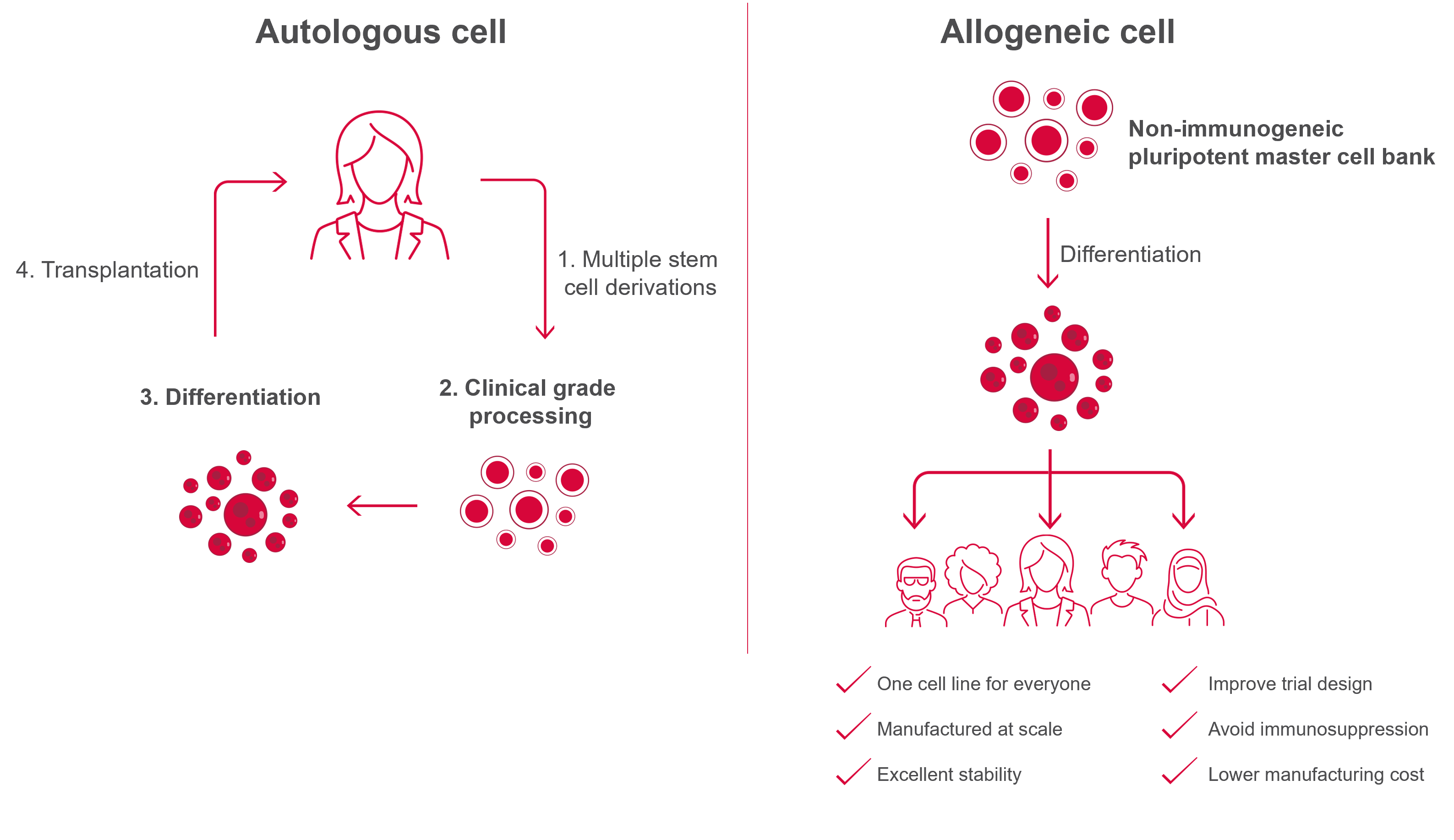
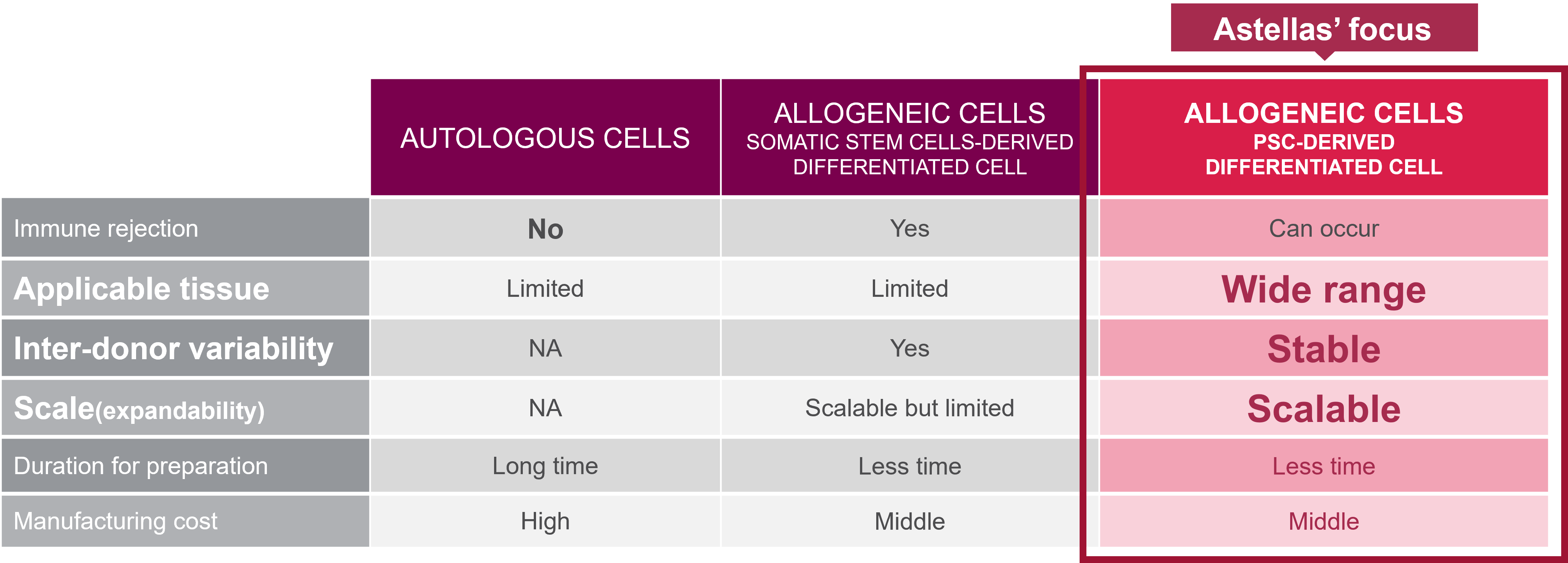
Pluripotent stem cell (PSC)-derived allogeneic cell therapy has the following advantages compared to autologous cell therapy
- Can be applied to any type of tissue: PSCs can differentiate into a wide range of cell types, allowing for the development of allogeneic cell therapies that can treat a variety of diseases or conditions
- Less inter-donor variability: Allogeneic cell therapy using cells from a consistent source avoids variability in cell quality and efficacy that occurs in autologous cell therapy
- Scalable: PSCs can be expanded in large numbers, allowing for the manufacture of large quantities of cells
- Less time for preparation: “Off-the-shelf” allogeneic cells differentiated from PSCs can be delivered to patients more quickly than autologous cells that require lengthy obtaining, differentiation, and expansion processes
- Low manufacturing cost: The scalability of PSCs and the ability to produce large quantities of cells can result in lower manufacturing costs, making the therapy more accessible for patients
However, when allogeneic cells are transplanted into a patient, the immune response can cause the transplanted cells to be eliminated from the body through immune rejection.
Our approaches for avoiding immune rejection are entering this field of therapy from the ophthalmology area, where immune rejection is less likely to occur, and utilizing Universal Donor Cell technology to avoid immune rejection.
Unique Capabilities in Cell Therapy
Universal Donor Cell technology
A unique technology for lowering the immune rejection potential of PSCs as a cornerstone capability for creating allogeneic therapies. Gene edits with a recombinant adeno-associated virus (AAV) knock out the expression of HLA class I and class II surface proteins that are thought to be primarily responsible for eliciting T-cell responses and immune rejection. Other gene edits lead to the expression of specific nonpolymorphic HLA molecules to provide essential class I signals that block lysis by NK cells.
Link to Universal cells Universal Cells
End to End expertise
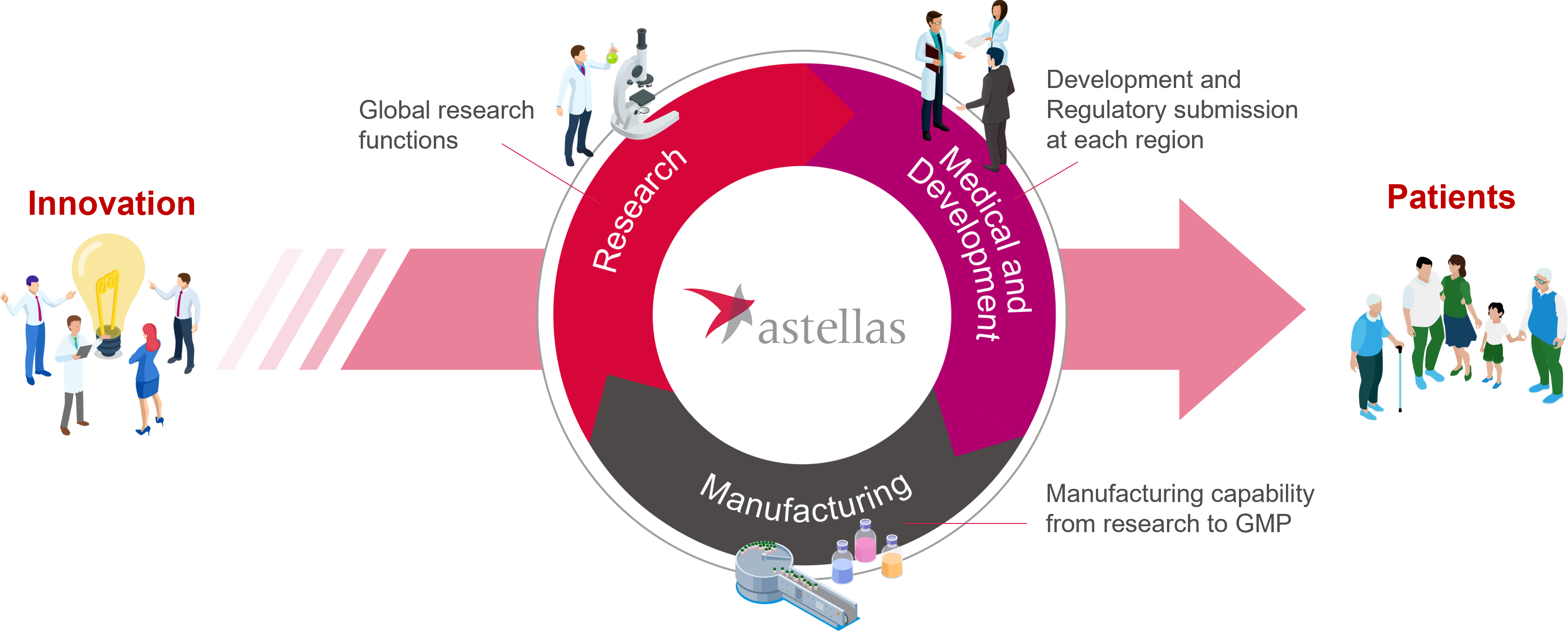
We have a variety of capabilities needed to bring innovations in cell therapy to patients. Pluripotent stem cell sourcing, Universal Donor Cell technology, gene editing and differentiation are key to our activities. We then apply expertise in standardization, quality assurance and knowledge of GMP manufacturing, freeze/thaw technology and formulation to move from the lab through to manufacturing cells for clinical use. In cell therapy "process is product" and it is important to assure the standard and quality of cell products. Astellas conducts R&D as one team with each function having a common goal under the R&D operating model.
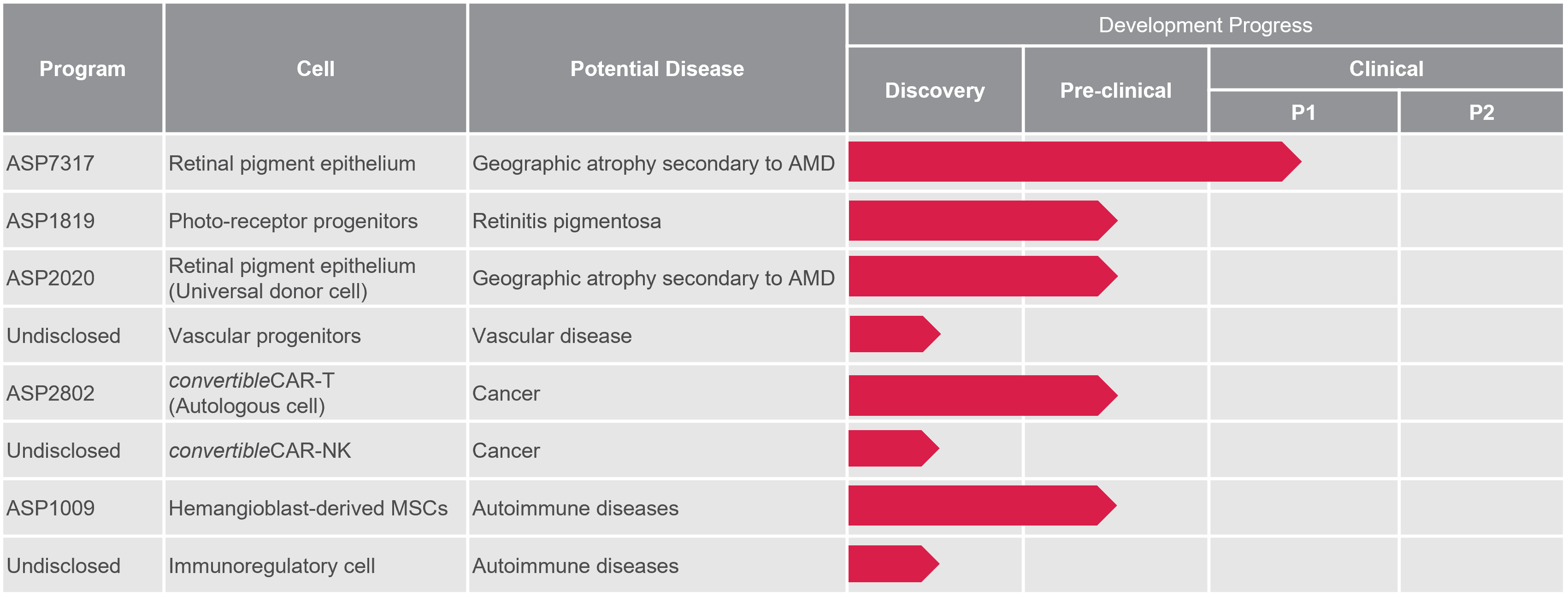
Our unique R&D strategy enables us to build a robust, competitive pipeline
Our most clinically-advanced program (ASP7317) is looking at the use of retinal pigmented epithelium cells for improving vision in patients with dry age-related macular degeneration. We also have several other ocular cell types in our portfolio, such as Photoreceptor Cells, Corneal Endothelial Cells, as well as HMC administered systemically. We are also developing next-generation cancer cell therapy utilizing state-of-the-art technologies. By combining multiple cutting-edge technologies, we believe we can create a game-changing cell therapy that is allogeneic, can attack multiple cancer antigens in a single-type cell, and can even destroy solid tumors.
Accurate as of Sep. 2023
CAR-T: Chimeric Antigen Receptor T cell, FcR: Fc receptor, NK: Natural Killer cell, MSC: Mesenchymal Stem Cell, AMD: Age-related Macular Degeneration

Message from AIRM President
Masahide Goto, Ph.D.
President
Astellas Institute for Regenerative Medicine
Cell therapy has long held great promise for patients with diseases for which there are few treatments or no cures. With recent advances in science, cell therapy is on the verge of becoming a reality for these unmet medical needs. At Astellas, we have made significant investments in building the capabilities, assets, talent, expertise, technology, and targeted portfolio needed to help realize its potential. However, due to the complexity of this field, we still need to deal with some significant scientific and administrative challenges. To overcome them, we need to incorporate cutting-edge science from our world-class internal team, as well as develop relationships with outside experts and collaborators in the biopharmaceutical ecosystem. By bringing together these top minds within cell therapy, we believe that we will be able to provide VALUE for patients and their families. If you feel the same, we’d like to hear from you.
Related Links