To administer a drug candidate on humans in clinical trials and bring it to market, it is crucial to have high-quality and reliable data from non-clinical studies. These studies include pharmacology, pharmacokinetic, and toxicology studies using cells and animals. Regulatory science plays a critical role in obtaining and evaluating these non-clinical data. The thorough evaluation enables research to maximize the value drug candidates deliver to patients while mitigating their risks. This leads to smoother regulatory approvals in each country, which are required for initiating clinical trials and commercializing the medicine.
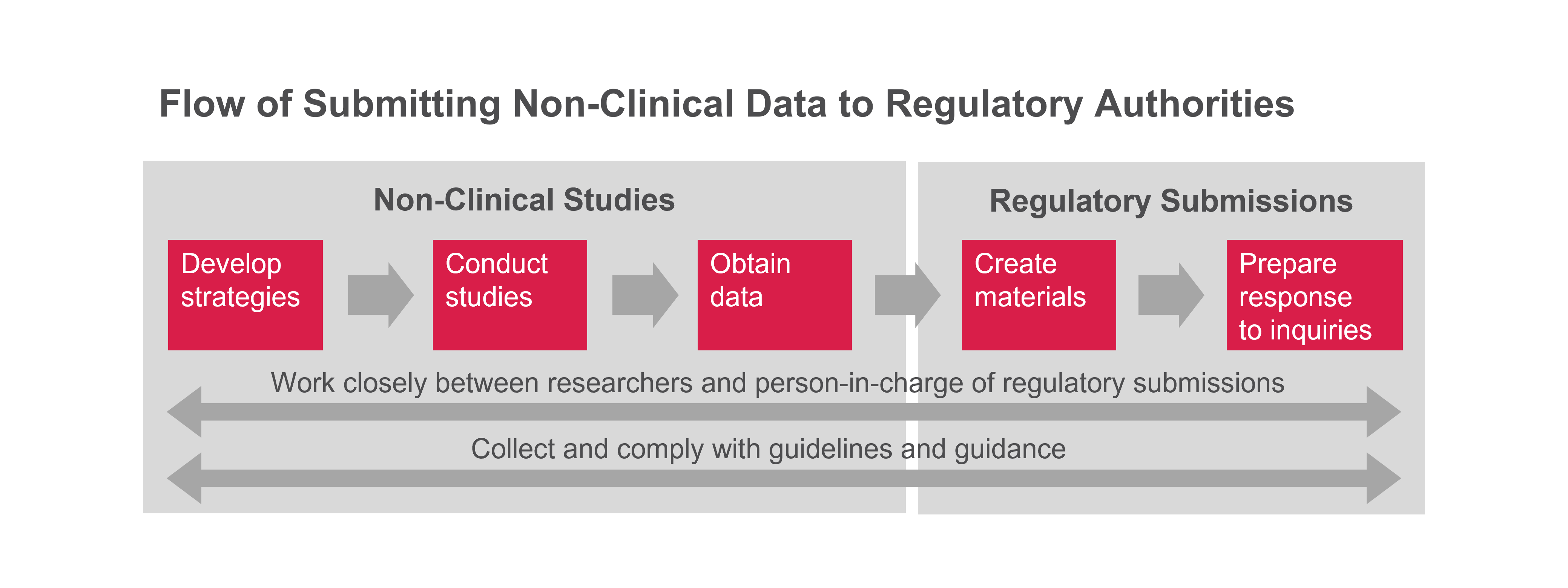
At Astellas, the research division covers a wide range of activities related to non-clinical aspects of regulatory submissions based on scientific evidence. This involves planning and conducting non-clinical studies, as well as creating documents based on the results. Our strategic approach to non-clinical studies allows us to submit the data to the regulatory agency on schedule in order to deliver new therapies to patients as quickly as possible.
Strategies for Obtaining High Quality and Reliable Non-Clinical Data
- Plan and conduct high quality and reliable non-clinical pharmacology, pharmacokinetics, and toxicology studies base on scientific evidence
- Plan and execute strategies for optimal non-clinical studies depending on the research and development stage
- Obtain non-clinical data to conduct safe and smooth clinical trials
When a drug candidate advances to the preparatory stage for clinical trials, the first step is to build a strategy for non-clinical studies to assess its fundamental properties. Subsequently with careful consideration for the optimal study design and procedures, non-clinical studies are conducted, ensuring transparency and consistency. Complying with regulatory guidelines is imperative to guarantee accuracy and objectivity throughout these studies. Statistical methods are used to confirm the reliability of the interpretation of data.
Through these properly managed non-clinical studies, we can obtain highly reliable data, forming a solid foundation for ensuring patient safety.
Regulatory Submissions
- Create content related to non-clinical studies as part of the application materials submitted to regulatory authorities. This includes the following activities:
- Investigational New Drug (IND) application
- New Drug Application/Biologic License Application (NDA/BLA)
- Prepare the response to non-clinical inquiries from the authorities
- Collect regulatory and guideline information for each country
Pharmaceutical companies interact with regulatory authorities throughout a drug lifecycle, from the time before first-in-human clinical trials to post-marketing.
We provide documented data from non-clinical studies to demonstrate the safety and efficacy for the drug candidate or product based on scientific evidence. Additionally, we use highly reliable data and advanced insights to create responses to inquiries from regulatory authorities globally.
Furthermore, we collect the latest regulatory information and conduct non-clinical studies in compliance with global guidelines and guidance to ensure prompt submissions to regulatory authorities.