-Total Solution for Early Detection of Atrial Fibrillation Using
Disposable Holter ECG device and AI-Based Analysis Service-
TOKYO, OSAKA, and IWATE, September 2nd, 2021 - Astellas Pharma Inc. (TSE: 4503, President and CEO: Kenji Yasukawa, Ph.D., “Astellas” ), Nitto Denko Corporation (TSE: 6988, President; Hideo Takasaki, “Nitto”), and M. Heart Co., Ltd. (President and CEO: Yoshimi Mizunuma, “M. Heart”) today announced that the three companies concluded a memorandum of understanding on September 1st concerning an ECG testing service.
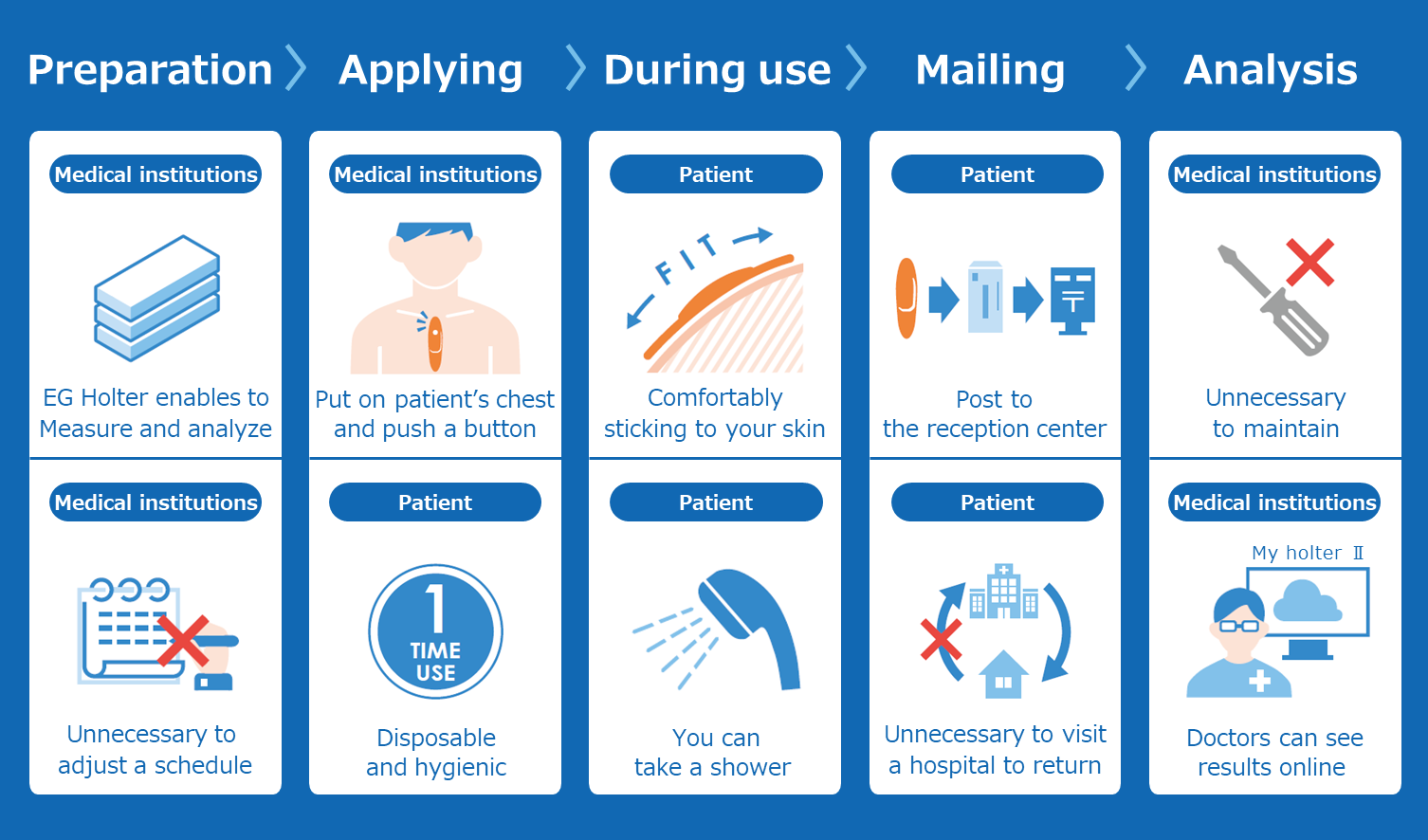
Based on this memorandum of understanding, Astellas, Nitto, and M. Heart will continue to look into Nitto developing and manufacturing the “EG Holter” a novel disposable holter ECG device, and Astellas exclusively selling it in Japan. M. Heart will play the role of marketing authorization holder of the EG Holter following from its background of providing development support and regulatory affairs consultations and be responsible for providing the service that will analyze the data obtained from the EG Holter through “MYHOLTER II,” a software for the Holter analyzer (“The ECG testing service”). In the future, Astellas will aim to provide EG Holter nationwide following pilot-sales.
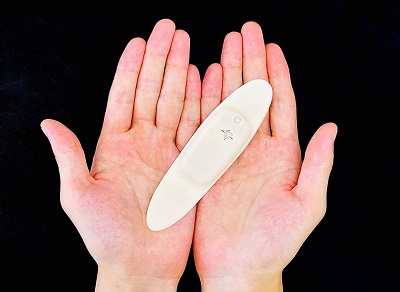
The EG Holter is disposable and hygienic. It is 6 millimeters thick,11 grams in weight, has no cords, and is waterproof (IPX4). Patients thus can wear it more comfortably. M. Heart obtained certification for the EG Holter as a medical device (Class II) in August 2021.
Astellas and M. Heart have developed “MYHOLTER II*1 jointly and built it into M. Heart’s cloud-type Holter ECG analysis service*2 in July for provision to healthcare professionals. MYHOLTER II is a system that analyzes Holter ECG data efficiently with high accuracy using unique artificial intelligence (AI). With the ECG testing service, after measurement, patients return it to the reception center by postal mail, eliminating the need to return the equipment back to a medical institution. Medical institutions can also see results online via MYHOLTER II, resulting in the reducing of the burden on both patients and medical institutions.
Medical costs for heart diseases and other cardiovascular diseases in Japan exceed 6 trillion yen per year—the highest of any disease category1. Of particular concern is cardiogenic cerebral embolism, which occurs when a thrombus that formed in the heart blocks an artery in the brain or neck. This has a high mortality rate (20%) and often results in severe sequelae, such as a bedridden state (40%)1. Atrial fibrillation is said to be the cause of cardiogenic cerebral embolism in 3 out of 4 cases2. Early detection of atrial fibrillation is an important social issue.
By providing a total solution that involves the combining of convenient ECG testing using the EG Holter and the effective and highly accurate data analysis using MYHOLTER II, Astellas, Nitto, and M. Heart are hoping that by promoting early detection and appropriate treatment of atrial fibrillation, a condition estimated to affect approximately 700,000 patients in Japan2 they can contribute to the prolongation of healthy life expectancy.
Click below for a copy of the full press release