TOKYO, October 28, 2020 - Astellas Pharma Inc. (TSE: 4503, President and CEO: Kenji Yasukawa, Ph.D., “Astellas” ) announced today that the United States (“U.S.”) Food and Drug Administration (FDA) has granted Fast Track designation based on nonclinical and clinical data for the development of ASP5354, an imaging agent being investigated for intraoperative ureter visualization in patients undergoing minimally invasive and open abdominopelvic surgeries. The FDA's Fast Track Designation Program aims to expedite the development and review of potential treatments for serious or potentially life-threatening illnesses with high unmet medical needs. The ASP5354 Fast Track designation is expected to faciliate development and potentially early clinical availability with faster reviews of the novel agent.
ASP5354 is an optical imaging agent being developed by Astellas' Rx+® business1 as a surgical adjunct to lessen the likelihood of iatrogenic ureteral injury (IUI) during complex abdominal and pelvic surgeries such as colorectal or gynecologic surgeries. IUI can result in long-term complications such as ureteral stricture / obstruction, ureteral-vaginal fistulae, acute or chronic renal failure, or sepsis. A survey of more than 2 million surgical cases in the U.S. shows that IUI is accompanied by higher rates of morbidity and, in some cases, mortality. Additionally, because of the need for potentially extensive reconstructive surgeries and extended hospitalizations, the costs to manage IUI can be exceedingly high2.
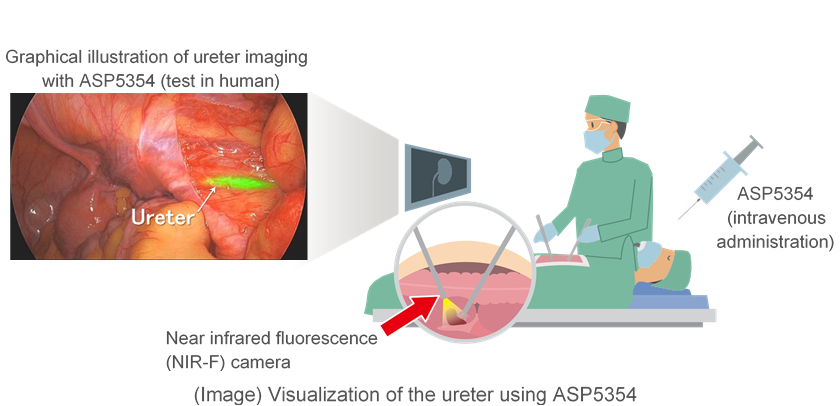
ASP5354 is a derivative of indocyanine green (ICG) that fluoresces upon excitation with a particular wavelength of near-infrared light. When administered intravenously, ASP5354 is primarily and rapidly excreted by the kidneys and provides the surgeon with visualization of the ureter(s) during surgery through the use of a near-infrared fluorescence (NIR-F) medical device. ASP5354 is an investigational compound discovered by Mie University and Nagoya University, with Astellas acquiring exclusive development and marketing rights worldwide.
In the Phase 1 study ASP5354 was safe and well tolerated at all doses evaluated in healthy volunteers. A Phase 2 study is currently underway to evaluate the safety and efficacy of ASP5354 in patients undergoing colorectal surgery. For more information on ASP5354 clinical trials, please visit www.clinicaltrials.gov.
Through its Rx+® business, Astellas aims to realize a society where people can live in their own way, both physically and mentally through scientifically based healthcare solutions. Through this venture, Astellas aims to optimize treatment by improving diagnostic accuracy and surgical outcomes. The development of ASP5354 for precision surgery is part of this effort. Going forward, Astellas looks forward to providing various solutions that contribute to more precise and safer surgical methods through the fusion of medical device innovation and pharmaceutical technology.
Click below for a copy of the full press release