Amgen Astellas BioPharma K.K. (Headquarters, Tokyo; President and Representative Director: Steve Sugino, “Amgen Astellas”) and Astellas Pharma Inc. (Headquarters, Tokyo; President and CEO: Yoshihiko Hatanaka, “Astellas”) today announced the launch of Repatha® SC Injection 420 mg Auto Mini Doser (AMD), an additional dosage formulation to Repatha® SC Injection 140 mg Syringe and Pen. Repatha® is the first proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor in Japan for the treatment of patients with familial hypercholesterolemia or hypercholesterolemia who have high risk of cardiovascular events and do not adequately respond to HMG-CoA reductase inhibitors (statins).
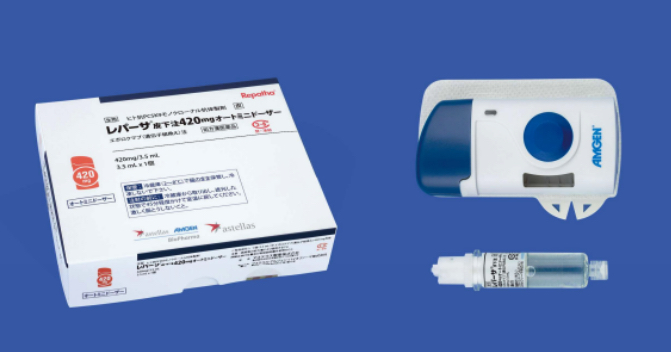
The new Repatha® SC Injection 420 mg AMD is a new dosage form consisting of a compact, palm-sized device with prefilled cartridge and a dedicated injection system. While the conventional syringes and pens required three subcutaneous injections of 140 mg to administer 420 mg, Repatha® SC Injection 420 AMD delivers a single dose in 9 minutes with a hands-free delivery system, allowing the patient to walk, stretch their arms, bend at the waist, or do other moderate everyday activities. Another characteristic of the product design is the needle which stays hidden during administration.
“For patients with high risk of cardiovascular events despite treatment with widely used statins, Repatha® provides an important treatment option,” said Tamio Teramoto, M.D., Ph.D., Director of Teikyo Academic Research Center. “While the pen device is already an elegant and friendly mode of administration for Repatha® , the new AMD is a welcome development not only because it further improves convenience for patients but it will also help alleviate the emotional stress that many patients have felt of piercing themselves with a needle.”
Repatha® SC Injection 420 mg Auto Mini Doser (AMD)
Repatha® (evolocumab) is a human monoclonal antibody that inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9). Repatha® binds to PCSK9 and inhibits circulating PCSK9 from binding to the low density lipoprotein (LDL) receptor (LDLR), preventing PCSK9-mediated LDLR degradation and permitting LDLR to recycle back to the liver cell surface. By inhibiting the binding of PCSK9 to LDLR, Repatha® increases the number of LDLRs available to clear LDL from the blood, thereby lowering LDL-C levels.
Repatha® is approved in more than 50 countries, including the U.S., Japan, Canada and in all 28 countries that are members of the EU. Applications in other countries are pending.
Important Japan Product Information
Repatha® SC Injection [generic name evolocumab (genetically recombination)] has the following indications.
Indications
Familial hypercholesterolemia, Hypercholesterolemia Only when patients who have high risk in cardiovascular events and do not adequately respond to HMG-CoA reductase inhibitors
Precautions Related to Indications
- Prior to Repatha therapy, patients should undergo adequate medical examination and tests to confirm a diagnosis of familial hypercholesterolemia or hypercholesterolemia.
- In patients with non-familial hypercholesterolemia, the use of Repatha should be considered for patients with high risk of cardiovascular events based on confirmed risk factors e.g. comorbid conditions including coronary artery disease, non-cardiogenic cerebral infarction, peripheral arterial disease, diabetes mellitus and chronic renal disease or medical history
Dosage and Administration
Heterozygous Familial Hypercholesterolemia and Hypercholesterolemia:
[Repatha® SC Injection 140 mg Syringe/Pen]
In general, for adults, evolocumab (genetically recombination) of 140 mg is administrated every 2 weeks or evolocumab of 420 mg is administrated every 4 weeks subcutaneously.
[Repatha® SC Injection 420 mg AMD]
In general, for adults, evolocumab (genetically recombination) of 420 mg is administrated every 4 weeks subcutaneously.
Homozygous Familial Hypercholesterolemia:
In general, for adults, 420 mg of evolocumab (genetical recombination) is administrated subcutaneously every 4 weeks. In case of insufficient response, 420 mg of evolocumab can be administered subcutaneously every 2 weeks. If evolocumab is administered as adjunctive therapy for patients with LDL apheresis, as starting dose, 420 mg of evolocumab can be administered subcutaneously every 2 weeks.
Precautions Related to Dosage and Administration
Repatha should be administered in combination with HMG-CoA reductase inhibitor-based lipid lowering therapy. [The efficacy and safety of Repatha monotherapy in Japanese patients have not been established.]
* For more information, please see the latest Japan Prescribing Information.
* The Notification #1215 (issued December 15, 2017) issued by Medical Affairs Division of Ministry of Health, Labour and Welfare sets forth the points to be considered when using this drug under the coverage of the National Health Insurance.
About Amgen Astellas BioPharma
Amgen Astellas BioPharma K.K.(http://www.aabp.co.jp/jp/) is a Japanese company that began operations on October 1, 2013, to provide breakthrough-science-based medicines to help address unmet medical needs of patients in Japan. The company is a joint venture between Amgen, one of the world’s leading independent biotechnology companies, and Astellas Pharma Inc., a leading Tokyo-based R&D oriented global pharmaceutical company.
AABP has grown into an organization with over 400 employees and comprehensive functions to be fully operational as a marketing authorization holder in Japan. The joint venture will become a wholly-owned Amgen affiliate as soon as 2020.
About Amgen
Amgen is committed to unlocking the potential of biology for patients suffering from serious illnesses by discovering, developing, manufacturing and delivering innovative human therapeutics. This approach begins by using tools like advanced human genetics to unravel the complexities of disease and understand the fundamentals of human biology.
Amgen focuses on areas of high unmet medical need and leverages its expertise to strive for solutions that improve health outcomes and dramatically improve people's lives. A biotechnology pioneer since 1980, Amgen has grown to be one of the world's leading independent biotechnology companies, has reached millions of patients around the world and is developing a pipeline of medicines with breakaway potential.
For more information, visit www.amgen.com and follow us on www.twitter.com/amgen.
About Astellas
Astellas Pharma Inc., based in Tokyo, Japan, is a company dedicated to improving the health of people around the world through the provision of innovative and reliable pharmaceutical products. We focus on Urology, Oncology, Immunology, Nephrology and Neuroscience as prioritized therapeutic areas while advancing new therapeutic areas and discovery research leveraging new technologies/modalities. We are also creating new value by combining internal capabilities and external expertise in the medical/healthcare business. Astellas is on the forefront of healthcare change to turn innovative science into value for patients. For more information, please visit our website at https://www.astellas.com/en.
Forward-Looking Statements (Amgen)
This news release contains forward-looking statements that are based on the current expectations and beliefs of Amgen. All statements, other than statements of historical fact, are statements that could be deemed forward-looking statements, including estimates of revenues, operating margins, capital expenditures, cash, other financial metrics, expected legal, arbitration, political, regulatory or clinical results or practices, customer and prescriber patterns or practices, reimbursement activities and outcomes and other such estimates and results. Forward-looking statements involve significant risks and uncertainties, including those discussed below and more fully described in the Securities and Exchange Commission reports filed by Amgen, including our most recent annual report on Form 10-K and any subsequent periodic reports on Form 10-Q and current reports on Form 8-K. Unless otherwise noted, Amgen is providing this information as of the date of this news release and does not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise.
No forward-looking statement can be guaranteed and actual results may differ materially from those we project. Discovery or identification of new product candidates or development of new indications for existing products cannot be guaranteed and movement from concept to product is uncertain; consequently, there can be no guarantee that any particular product candidate or development of a new indication for an existing product will be successful and become a commercial product. Further, preclinical results do not guarantee safe and effective performance of product candidates in humans. The complexity of the human body cannot be perfectly, or sometimes, even adequately modeled by computer or cell culture systems or animal models. The length of time that it takes for us to complete clinical trials and obtain regulatory approval for product marketing has in the past varied and we expect similar variability in the future. Even when clinical trials are successful, regulatory authorities may question the sufficiency for approval of the trial endpoints we have selected. We develop product candidates internally and through licensing collaborations, partnerships and joint ventures. Product candidates that are derived from relationships may be subject to disputes between the parties or may prove to be not as effective or as safe as we may have believed at the time of entering into such relationship. Also, we or others could identify safety, side effects or manufacturing problems with our products, including our devices, after they are on the market.
Our results may be affected by our ability to successfully market both new and existing products domestically and internationally, clinical and regulatory developments involving current and future products, sales growth of recently launched products, competition from other products including biosimilars, difficulties or delays in manufacturing our products and global economic conditions. In addition, sales of our products are affected by pricing pressure, political and public scrutiny and reimbursement policies imposed by third-party payers, including governments, private insurance plans and managed care providers and may be affected by regulatory, clinical and guideline developments and domestic and international trends toward managed care and healthcare cost containment. Furthermore, our research, testing, pricing, marketing and other operations are subject to extensive regulation by domestic and foreign government regulatory authorities. Our business may be impacted by government investigations, litigation and product liability claims. In addition, our business may be impacted by the adoption of new tax legislation or exposure to additional tax liabilities. If we fail to meet the compliance obligations in the corporate integrity agreement between us and the U.S. government, we could become subject to significant sanctions. Further, while we routinely obtain patents for our products and technology, the protection offered by our patents and patent applications may be challenged, invalidated or circumvented by our competitors, or we may fail to prevail in present and future intellectual property litigation. We perform a substantial amount of our commercial manufacturing activities at a few key facilities, including in Puerto Rico, and also depend on third parties for a portion of our manufacturing activities, and limits on supply may constrain sales of certain of our current products and product candidate development. In addition, we compete with other companies with respect to many of our marketed products as well as for the discovery and development of new products. Further, some raw materials, medical devices and component parts for our products are supplied by sole third-party suppliers. Certain of our distributors, customers and payers have substantial purchasing leverage in their dealings with us. The discovery of significant problems with a product similar to one of our products that implicate an entire class of products could have a material adverse effect on sales of the affected products and on our business and results of operations. Our efforts to acquire other companies or products and to integrate the operations of companies we have acquired may not be successful. We may not be able to access the capital and credit markets on terms that are favorable to us, or at all. We are increasingly dependent on information technology systems, infrastructure and data security. Our stock price is volatile and may be affected by a number of events. Our business performance could affect or limit the ability of our Board of Directors to declare a dividend or our ability to pay a dividend or repurchase our common stock.
Cautionary Notes (Astellas)
In this press release, statements made with respect to current plans, estimates, strategies and beliefs and other statements that are not historical facts are forward-looking statements about the future performance of Astellas. These statements are based on management’s current assumptions and beliefs in light of the information currently available to it and involve known and unknown risks and uncertainties. A number of factors could cause actual results to differ materially from those discussed in the forward-looking statements. Such factors include, but are not limited to: (i) changes in general economic conditions and in laws and regulations, relating to pharmaceutical markets, (ii) currency exchange rate fluctuations, (iii) delays in new product launches, (iv) the inability of Astellas to market existing and new products effectively, (v) the inability of Astellas to continue to effectively research and develop products accepted by customers in highly competitive markets, and (vi) infringements of Astellas’ intellectual property rights by third parties. Information about pharmaceutical products (including products currently in development) which is included in this press release is not intended to constitute an advertisement or medical advice.
For information on this matter contact:
Corporate Affairs, Amgen Astellas BioPharma K.K. (TEL +81-3-5293-9694)
Corporate Communications, Astellas Pharma Inc. (TEL +81-3-3244-3201)


