TOKYO, OSAKA, and IWATE, June 30, 2022 – Astellas Pharma Inc. (TSE: 4503, President and CEO: Kenji Yasukawa, Ph.D., “Astellas” ), Nitto Denko Corporation (TSE: 6988, President: Hideo Takasaki, “Nitto”), and M. Heart Co., Ltd. (President and CEO: Yoshimi Mizunuma, “M. Heart”) today announced that the three companies have entered into an agreement for a sales pilot of disposable Holter ECG device "EG HolterTM" for the Japan market. Based on this agreement, Astellas has established an e-commerce site for healthcare professionals and will start the sales pilot of the device today.
This is Astellas’ first initiative in Rx+® program to promote a product through an e-commerce site. After verifying the business model through the sales pilot, Astellas will move to full-scale marketing of the product.
EG Holter is a holter electrocardiograph designed and developed by Nitto. Because it is a disposable device, it is hygienic and does not require maintenance. It is 6 mm thick,11 g in weight, has no cords, and is water resistance (IPX4). It is easy to attach and remove.
Data obtained by EG Holter will be analyzed by "MYHOLTER II", which is software for the holter analyzer jointly developed by Astellas and M. Heart. MYHOLTER II is a program that analyzes holter ECG data with a proprietary analytical algorithm using artificial intelligence (AI). M. Heart has obtained cerification for EG Holter and MYHOLTER II as medical device (Class II).
Astellas, Nitto and M. Heart have come together to create a simple, convenient, patient-centered solution for patients and medical institutions.
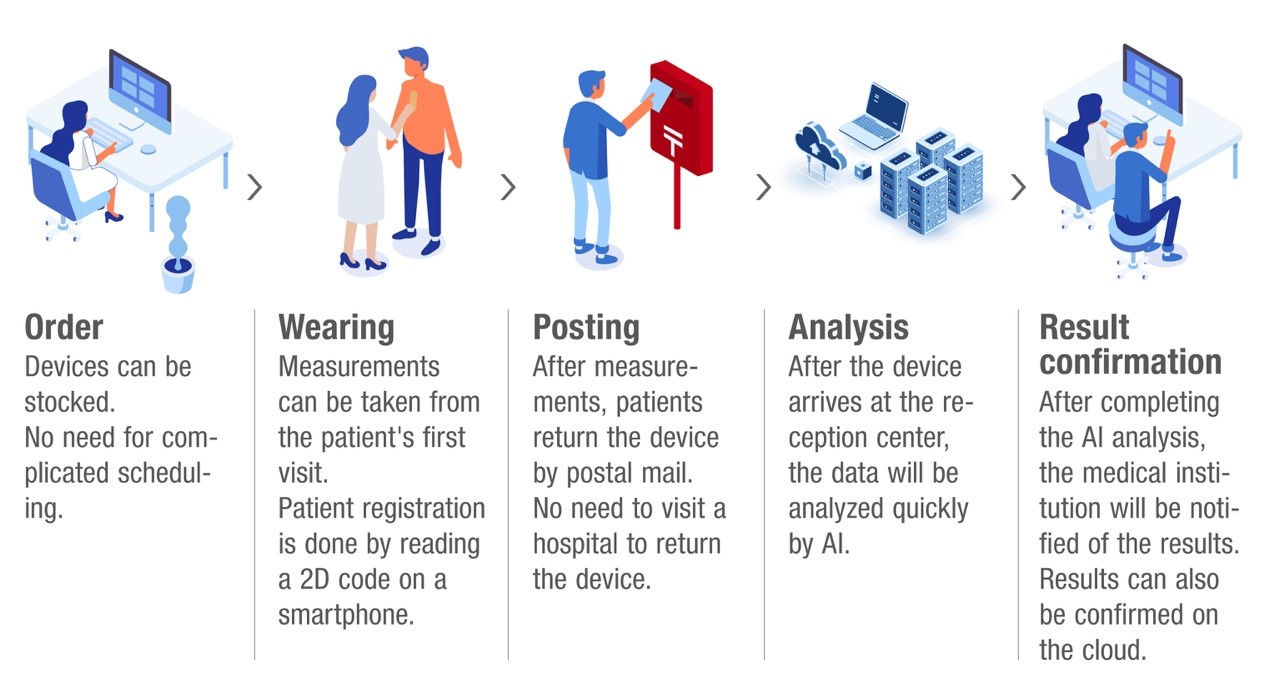
Analysis flow using EG Holter and MYHOLTER II
Medical costs for heart diseases and other cardiovascular diseases in Japan exceed 6 trillion yen per year―the highest of any disease category1. Of particular concern is cardiogenic cerebral embolism, which occurs when a thrombus that formed in the heart blocks an artery in the brain or neck. This has a high mortality rate (20%) and often results in severe sequelae, such as a bedridden state (40%)2. Atrial fibrillation is said to be the cause of cardiogenic cerebral embolism in 3 out of 4 cases2. Early detection of atrial fibrillation is an important social issue.
By providing a total solution that involves combining convenient ECG testing using the EG Holter and the data analysis using MYHOLTER II, Astellas, Nitto, and M. Heart propose to enable early detection and appropriate treatment of atrial fibrillation, a condition estimated to affect approximately 700,000 patients in Japan2, and contribute to the prolongation of healthy life expectancy.
Click below for a copy of the full press release